Abstract
Introduction
Myelodysplastic syndromes (MDS) are clonal hematopoietic stem cell disorders characterized mainly by ineffective hematopoiesis. Xenograft models of MDS in immunodeficient mice have been instrumental in improving our understanding of clonal origin and evolution of this disease (Mian & Rouault-Pierre et al., Nat Commun. 2015; Rouault-Pierre & Mian et al Haematologica 2016; Leukemia, 2017); however, the low level of engraftment generally observed in these mouse models makes them impractical for testing therapeutic strategies. We used a 3D humanized scaffold in vivo system to test the MDS stem cell engraftment as well as study the cross talk between MDS-Initiating cells (MDS-ICs) and their niche.
Methodology
In this study bone marrow (BM) cells from 31 patients (27 MDS cases covering all WHO subtypes, 1 CMML, 1 MDS/MPD and 2 sAML) were studied. Initially, whole-exome sequencing was performed on all cases. Colony-forming assay was used to assess the colony forming ability of BM CD34+ cells from each patient. Mesenchymal stromal cells (MSCs) were isolated from the BM of the patient samples. We then used 3 immunodeficient mouse models (NSG, NSG-SGM3 and NBSGW) to study the engraftment kinetics and clonal evolution of the transplanted cells in gelatin-based 3D scaffolds coated with patient MSCs (RARS/RCMD, autologous; RAEB, autologous/allogenic).
Results
BM MSCs obtained from the MDS patients showed variable expansion levels, with the ability to differentiate into the adipogenic lineage whereas the osteogenic differentiation was highly aberrant.
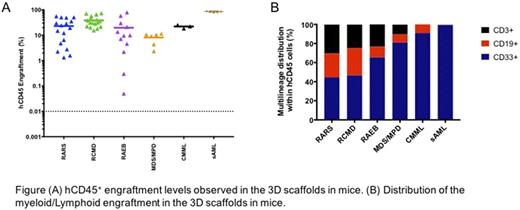
Next, 0.5x106 to 3x106 patient (n=31) mononuclear cells (MNCs T cell depleted) were seeded into 3D-scaffolds prior to implantation in NSG-SGM3 and/or NSG mice. We observed persistent long-term engraftment of hCD45+ cells ranging from 0.05% to 80% (up to 18 weeks). Importantly, 74% of the cases generated an engraftment level of >20% hCD45+ cells. Similar engraftment levels were observed in mice humanized scaffolds that were injected with BM cells from MDS/MPD, CMML and sAML (hCD45+ 10%, 22% and 86%, respectively). No difference in the hCD45+ cell engraftment between the patients with low-risk or high-risk MDS was observed.
Remarkably, our 3D humanized scaffolds generated multi-lineage engraftment, with mice that received BM cells from RAEB cases yielding higher myeloid engraftment compared to RARS and RCMD (RARS hCD33+ 44%; RCMD hCD33+ 46.6%; RAEB hCD33+ 65%). Furthermore, transplantation of BM cells from 5 patients into NBSGW mice gave rise to engraftment levels of hCD45+ cells similar (hCD45+ cells, up to 40%) to the NSG and NSG-SGM3 mice. The MDS cells recovered from the mice maintained their genotypic characteristics, as demonstrated by the presence of the mutations that remained largely unchanged between the pre- and post-transplanted samples.
Interestingly, Immuno-staining of the scaffolds revealed colonization by the host murine vasculature (mCD31+ endothelial cells). In line with this finding, mouse hematopoietic cells were also found to have migrated into the implanted humanized scaffolds. Surprisingly, we did not observe hCD45+ cell engraftment in the bone marrow of any of the xenotransplanted mouse strains (NSG or NSG-SGM3 mice with or without Irradiation and non-irradiated NBSGW mice). However, we demonstrated that MDS stem cells migrate by implanting a supplementary scaffold into NSG-SGM3 and NBSGW mice that was only coated with MDS-MSCs. Notably, these MDS-MSC seeded scaffolds were colonized by the circulating human MDS cells.
Finally, we tested whether the cells recovered from the primary engrafted 3D humanized scaffolds would be able to generate secondary transplants. Our data shows that these cells were able to engraft in the secondary mice implanted with humanized 3D scaffolds.
Discussion
Our model provides evidence that MDS BM cells are highly dependent on humanized MSC niche, confirming the importance of the cross-talk between MDS-ICs and MDS niche. Our data demonstrates that MDS stem cells do migrate out of the 3D Scaffold but require their "own niche" for colonization. Thus, our in vivo 3D model system, can be reliably used to better dissect the cross-talk between MDS stoma and MDS-ICs. This model requiring relatively low number of human MNCs, provides a robust preclinical assessment tool to study clonal evolution as well as to test therapeutic strategies on the MDS clonality prior to treatment of MDS patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.